Answer:
-) 3-bromoprop-1-ene
-) 2-bromoprop-1-ene
-) 1-bromoprop-1-ene
-) bromocyclopropane
Step-by-step explanation:
In this question, we can start with the I.D.H (hydrogen deficiency index):
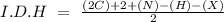
In the formula we have 3 carbons, 5 hydrogens, and 1 Br, so:
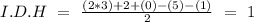
We have an I.D.H value of one. This indicates that we can have a cyclic structure or a double bond.
We can start with a linear structure with 3 carbon with a double bond in the first carbon and the Br atom also in the first carbon (1-bromoprop-1-ene). In the second structure, we can move the Br atom to the second carbon (2-bromoprop-1-ene), in the third structure we can move the Br to carbon 3 (3-bromoprop-1-ene). Finally, we can have a cyclic structure with a Br atom (bromocyclopropane).
See figure 1
I hope it helps!