Answer:
Volume of
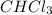 = 15.31 mL
= 15.31 mL
Volume of
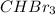 = 4.69 mL
= 4.69 mL
Step-by-step explanation:
Given that:
the density of the mixture = 1.82 g/mL
From the density of the pure samples
The density of
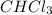 = 1.492 g/mL
= 1.492 g/mL
The density of
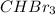 = 2.890 g/mL
= 2.890 g/mL
The total volume of the liquid mixture = 20.0 mL
Suppose the volume of
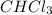 = P ml
= P ml
and the volume of
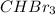 = Q ml
= Q ml
the sum of their volumes should be equal to the total volume of the mixture
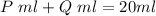 ----- (1)
----- (1)
However, we know that Density = mass/volume
∴ mass = density × volume
The equation can now be expressed as:
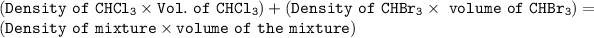
1.492 g/mL × P mL + 2.890 g/mL × Q mL = 1.82 g/mL × 20 mL ---- (2)
From equation (1) ;
let Q = 20 - P
The replace the value of P into equation (2)
1.492 g/mL × P mL + 2.890g/mL × (20 - P) mL = 1.82 g/mL × 20 mL
1.492 P g + 57.8g - 2.890 P g = 36.4g
1.492 P g - 2.890 P g = 36.4g - 57.8g
-1.398 P g = -21.4g
P = -21.4g/-1.398g
P = 15.31 mL
Q = 20 - P
Q = (20 - 15.31) mL
Q = 4.69 mL
∴
Volume of
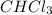 = 15.31 mL
= 15.31 mL
Volume of
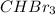 = 4.69 mL
= 4.69 mL