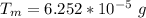Complete Question
What mass of aluminum has a total nuclear charge of 2.9 C?
Aluminum has atomic number 13. Suppose the aluminum is all of the isotope with 14 neutrons.
Answer:
The mass is
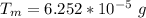
Step-by-step explanation:
From the question we are told that
The total nuclear charge is
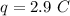
The atomic number is
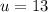
The number of neutron is
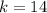
Generally the number of positive charge is mathematically represented as
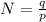
here p is the charge on a single proton with value
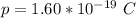
So
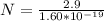
=>
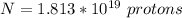
Now since 1 atom contains 13 proton
The number of atoms present is
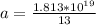
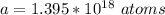
Then the number of moles present is mathematically represented as
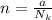
Where N_k is the Boltzmann constant with value
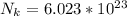
So
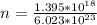
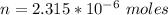
Generally one mole of aluminum is equal to 27 g
So
The total mass of aluminum is
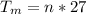
=>
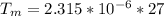
=>