Given :
Concentration of NaCl ,
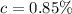 .
.
To Find :
Th amount of solid NaCl must be weighed out on the balance to prepare a bottle full containing 3500 ml .
Solution :
Concentration of NaCl is 0.85 % .
It means , 0.85 gm of NaCl in 100 ml of water .
So , amount of solid NaCl required to prepare 3500 ml solution is :
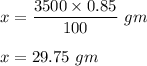
Therefore , amount of solid NaCl required is 29.75 gm .
Hence , this is the required solution .