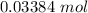Answer:
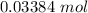
Step-by-step explanation:
Step 1: Determine important information
Ideal gas law →

At the end of the problem statement we can see that the pressure is
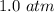 . V is the volume which is given as
. V is the volume which is given as
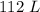 . n is the amount of substance which is what we are trying to find. R is the ideal gas constant is the same for every problem which is
. n is the amount of substance which is what we are trying to find. R is the ideal gas constant is the same for every problem which is
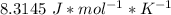 . Finally, T is the temperature which is given as
. Finally, T is the temperature which is given as
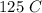 but we have to convert to kelvins which we get
but we have to convert to kelvins which we get
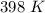 .
.
Step 2: Plug in the information and solve

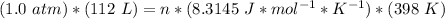
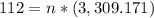
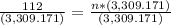
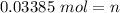
Answer: