Answer:
Step-by-step explanation:
For pH of a buffer solution , the equation is
pH = pKa + log [ A⁻ ] / [ HA ]
Putting the values
7 = - log 56 x 10⁻¹⁰ + log [ NH₃ ] / [ NH₄⁺]
7 = 10 - log 56 + log [ NH₃ ] / [ NH₄⁺]
- 3 + 1.748 = log [ NH₃ ] / [ NH₄⁺]
log [ NH₃ ] / [ NH₄⁺] = - 1.25
[ NH₃ ] / [ NH₄⁺] =
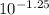
Required ratio =
2 )
In the formation of NH₄⁺ , generally HCl acid is required . A little bit of excess HCl will change the pH required . So , this mixture is a poor choice for getting pH 7 for a buffer solution .