Answer:
The volume of 40% solution= 4.3L
The volume of 5% solution= 10L - 4.3L= 5.7L
Explanation:
From the given information:
Let consider x to be the of volume 40% solution
therefore the volume of 5% is said to be:
10 L= 40% solution volume + 5% solution volume
10 L= (x + 5%) solution volume
making 5% solution volume the subject; we have
5% solution volume= 10 L - x
To estimate the value of the volume, we now have:
Volume of 20% × Concentration of 20%= (Volume of 40% × concentration40%) + (Volume of 5% × Concentration of 5% )
∴
10L × 20%= (x) L × 35% + 10 L × 5%
(x) L × 35 = 10L × 20 - 10 L × 5 = 10 L × 15
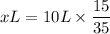
x = 4.28 L
x
 4.3L
4.3L
∴
The volume of 40% solution= 4.3L
The volume of 5% solution= 10L - 4.3L= 5.7L