Answer:
number of mole is 31342.36 moles
mass is 125.369 kg
Step-by-step explanation:
Diameter of the spherical balloon d = 9 m
radius r = d/2 = 9/2 = 4.5 m
The volume pf the sphere balloon ca be calculated from
V =
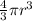
V =
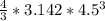 = 381.75 m^3
= 381.75 m^3
Temperature of the gas T = 20 °C = 20 + 273 = 293 K
Pressure of the helium gas = 200 kPa = 200 x 10^3 Pa
number of moles n = ?
Using
PV = nRT
where
P is the pressure of the gas
V is the volume of the gas
n is the mole number of the gas
R is the gas constant = 8.314 m^3⋅Pa⋅K^−1⋅mol^−1
T is the temperature of the gas (must be converted to kelvin K)
substituting values, we have
200 x 10^3 x 381.75 = n x 8.314 x 293
number of moles n = 76350000/2436 = 31342.36 moles
We recall that n = m/MM
or m = n x MM
where
n is the number of moles
m is the mass of the gas
MM is the molar mass of the gas
For helium, the molar mass = 4 g/mol
substituting values, we have
m = 31342.36 x 4
m = 125369.44 g
m = 125.369 kg