Answer:
A) 6.00 mol.
B) 0.375 L or 375 mL
C) 6.00 M
Step-by-step explanation:
Hello,
A) In this case, from the definition of molarity, we compute the moles for the given volume and concentration:
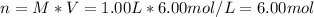
B) In this case, from the stock solution, the required volume is:
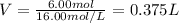
C) In this case, we apply the following formula for dilution process:

Thus, solving for the final molarity, we obtain:
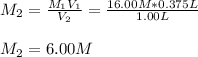
Regards.