Given :
a.
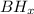 b.
b.
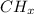 c.
c.
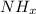 d.
d.
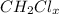 .
.
To Find :
Find the most likely vale of x for each one .
Solution :
a .
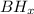
Because boron have valency of 3 .
So , x = 3 .
b .
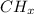
Valency of carbon is 4 .
x = 4 .
c .
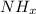
Valency of nitrogen is 3 .
Therefore , x = 3 .
d .
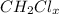
Now ,we know valency of carbon is 4 and hydrogen is 1 .
Also , two hydrogen are already there .
So , only 2 electrons left to share .
Since , chlorine have valency of 1 .
Therefore , only 2 electrons of chlorine can connect .
x = 2 .
Hence , this is the required solution .