Answer:
Compound A: 1-bromo-1-methylcyclohexane
Compound B: 1-methylcyclohex-1-ene
Step-by-step explanation:
In this question, we can start with the I.D.H (hydrogen deficiency index):
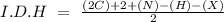
In the formula we have 7 carbons, 13 hydrogens, and 1 Br, so:
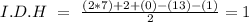
We have an I.D.H value of one. This indicates that we can have a cyclic structure or a double bond.
We have to keep in mind that the Br atom must be bonded to a tertiary carbon. We can not have a double bond because in the ozonolysis reaction we have only 1 product, therefore, we can not have a double bond in the initial molecule (if we have a double bond in the initial molecule we will have more than 1 product in the ozonolysis reaction).
With this in mind, we will have a cyclic structure. If we have 7 carbons and we need a tertiary alkyl halide. We can have a cyclic structure of 6 members and a methyl group bonded to a carbon that also is bonded to a Br atom (1-bromo-1-methylcyclohexane).
In the reaction with
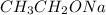 we will have an elimination reaction. In other words, we have the production of a double bond inside of the cyclic structure (1-methylcyclohex-1-ene).
we will have an elimination reaction. In other words, we have the production of a double bond inside of the cyclic structure (1-methylcyclohex-1-ene).
See figure 1 for further explanations.
I hope it helps!