Answer:
A) The value of the diameter in inches is 5.19685 × 10⁻⁹ inches
B) There are 1,590,909,091 atoms on a 21.0cm line
Step-by-step explanation:
A) To determine what its diameter is in inches
We will convert 1.32 × 10⁻¹⁰ m to inches
First convert this to centimeters (cm)
1.32 × 10⁻¹⁰m = 1.32 × 10⁻¹⁰ × 10² cm
= 1.32 × 10⁻⁸cm
Now, since 1 inch = 2.54 cm
∴ 1 cm =
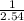 inches
inches
If 1 cm =
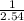 inches
inches
Then 1.32 × 10⁻⁸ cm will be
1.32 × 10⁻⁸ ×
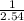 inches
inches
= 5.19685 × 10⁻⁹ inches
This is the value of the diameter in inches
B) For the number of atoms on a 21.0cm line
The diameter of 1 atom is 1.32 × 10⁻¹⁰m
1.32 × 10⁻¹⁰m = 1.32 × 10⁻⁸cm
Hence, on a 21.0 cm line, we will have
(21.0 / 1.32 × 10⁻⁸) atoms
= 1590909091 atoms
Hence, there are 1,590,909,091 atoms on a 21.0cm line