Answer:
See the answer below
Step-by-step explanation:
Using the dilution equation:
m1v1 = m2v2
m1 =100 μM, v1 = ?, v2 = 10 mL
In order to prepare 80 μM, then m2 = 80 μM
v1 =
 = 8 mL
= 8 mL
In order to prepare 60 μM
v1 =
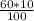 = 6 mL
= 6 mL
In order to prepare 40 μM
v1 =
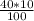 = 4 mL
= 4 mL
In order to prepare 20 μM
v1 =
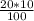 = 2 mL
= 2 mL
Since the final solution has to be up to the 10 mL mark, the complete table would be:
Target Concentration(μM) Volume of stock(mL) Volume of water(mL)
20 2 8
40 4 6
60 6 4
80 8 2