Answer:
- Take 3.3 mL of 3.0-M hydrochloric acid and subsequently add 76.7 mL of water to complete the 100.00 mL.
- Take 11.7mL of 6.0-M hydrochloric acid and subsequently add 88.3 mL of water to complete the 100.00 mL
Step-by-step explanation:
Hello,
In this case, given that the dilutions are preparedfrom 3.0-M and 6.0-M hydrochloric acid, we must proceed as follows:
- 3.0-M stock: when using this stock, the aliquot you must take is computed as shown below:
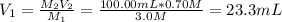
It means that you must take 23.3 mL of 3.0-M hydrochloric acid and subsequently add 76.7 mL of water to complete the 100.00 mL.
- 6.0-M stock: when using this stock, the aliquot you must take is computed as shown below:
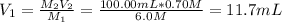
It means that you must take 11.7mL of 6.0-M hydrochloric acid and subsequently add 88.3 mL of water to complete the 100.00 mL.
Regards.