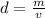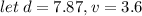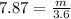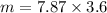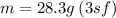Answer:
The mass of iron shot is 28.3 grams.
Step-by-step explanation:
Given that the initial volume of water is 47.5 mL and final volume of water containing iron shot is 51.1mL. First, you have to find the volume of iron shot by subtracting :
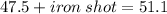
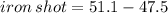
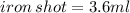
Next, we have to apply density formula, D = mass/volume :