Answer:
4.14516129 g/mL
Step-by-step explanation:
The density can be found with the following formula.
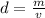
where
 is the mass and
is the mass and
 is the volume.
is the volume.
We know the mass is 25.7 grams. We must find the volume.
The volume is equal to the volume of water that is displaced. Subtract the initial volume from the final volume.
⇒ final volume - initial volume
The initial volume is 35.5 mL and the final volume is 41.7 mL.
⇒ 41.7 mL - 35.5 mL
⇒ 6.2 mL
The volume of the aluminum piece is 6.2 mL.
Now we know the mass and the volume.
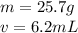
Substitute the values into the formula.
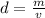
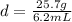
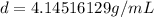
The density of the aluminum sample is 4.14516129 g/mL