Answer:
The volume of first and second compound are 9.15 ml and 10.85 ml.
Step-by-step explanation:
Given that,
Density of graphite = 2.25 g/cm³
Volume of mixture = 20.0 mL
Density of first compound = 1.492 g/ml
Density of second compound = 2.890 g/ml
Let the volume of first mixture = x
The volume of second mixture = (20-x)....(I)
We need to calculate the volume of first compound
Using formula of density of mixture
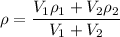
Where,
 = volume of first compound
= volume of first compound
 = volume of second compound
= volume of second compound
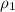 =density of first compound
=density of first compound
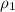 = density of first compound
= density of first compound
Put the volume into the formula
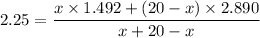
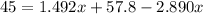
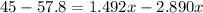
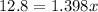
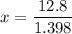
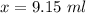
We need to calculate the volume of second compound
Using equation (I)
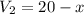
Put the value of x
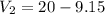
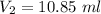
Hence, The volume of first and second compound are 9.15 ml and 10.85 ml.