Answer:
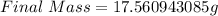
Step-by-step explanation:
Given
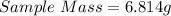
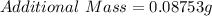
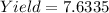
Required
Determine the final mass
First we need to determine the total mass after the sample mass is added to an additional sample;
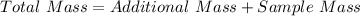
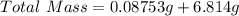
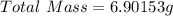
Next, divide the total mass by 3
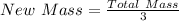
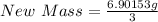
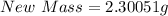
The final mass is calculated as follows;
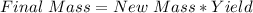
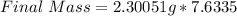
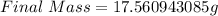
Hence, the final mass is 17.560943085g