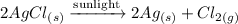Answer: 2) see below
3) 2AgCl → 2Ag + Cl₂
Step-by-step explanation:
2)
Step 1: Crushing & Grinding CuFeS₂(s)→ CuFeS₂(s)
Step 2: Froth Flotation CuFeS₂(s)----> CuFeS₂ (l)
Step 3: Roasting 2CuFeS₂(l) + 3O₂(g) → 2FeO(s) + 2CuS(s) + 2SO₂(g)
Step 4: Converting matte to blister Cu₂S(l) + O₂(g) → 2Cu(l) + SO₂(g)
Step 5: Anode Casting Cu(l) → Cu(s)
Step 6: Electrolytic Refining Cu(s) → Cu(s)
Anode: Cu(s) → Cu₂ + (aq) + 2e-
Cathode: Cu2 + (aq) + 2e- → Cu(s)
see diagram below for illustration of steps
3) When silver chloride (solid) is exposed to sunlight, it decomposes into silver (solid) and chlorine (gas).
The equation is written as: