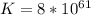Answer:
The value is
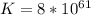
Step-by-step explanation:
From the question we are told that
The equation is
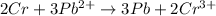
The temperature is
![T = 25^oC = 298 K [room \ temperature ]](https://img.qammunity.org/2021/formulas/chemistry/college/oz59jx2ogdehps8tyk4nbppmnhzvtruw7v.png)
The emf at standard condition is
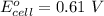
Generally at the cathode
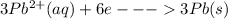
At the anode
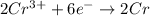
Generally for an electrochemical reaction, at room temperature the Gibbs free energy is mathematically represented as
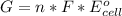
Here n is the no of electron with value n = 6
F is the Faraday's constant with value 96487 J/V
=>
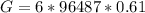
=>
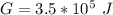
This Gibbs free energy can also be represented mathematically as
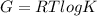
Here R is the cell constant with value 8.314J/K
K is the equilibrium constant
From above
=>
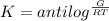
Generally antilog = 2.718
=>
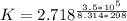
=>