Answer:
1.
Step-by-step explanation:
Hello,
In this case, for the given reaction we first assign the oxidation state for each species:
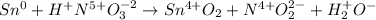
Whereas the half reactions are:
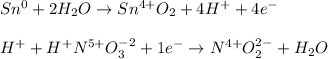
Next, we exchange the transferred electrons:
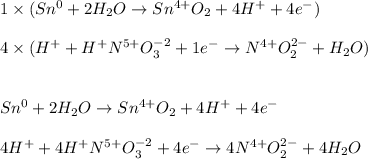
Afterwards, we add them to obtain:
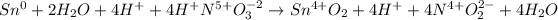
By adding and subtracting common terms we obtain:
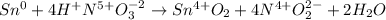
Finally, by removing the oxidation states we have:
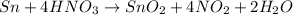
Therefore, the smallest whole-number coefficient for Sn is 1.
Regards.