Answer:
60% and 52%
Step-by-step explanation:
At Standard temperature and volume (STP)
Volume of gas = 22.4L
Density = mass/volume
⇒ 1.186 =
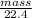
= 26.566g
Molar mass of O2 = 16g/mole
Molar mass of N2 = 14g/mol
% mass of O2 = 16/26.566 × 100
= 60.23 %
% mass of N2 = 14/26.566 × 100
= 52.70 %
(Check: totl composition of gas is 100% i.e 60 + 52 %)