Answer:
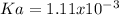
Step-by-step explanation:
Hello,
In this case, since the ionization of the given HA acid is:
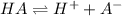
The equilibrium expression is:
![Ka=([H^+][A^-])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/kzt7pcnoonz2fwfrgadywivsijtflo2dmk.png)
Whereas the concentration of hydrogen ions is compute from the pH=
![[H^+]=10^(-pH)=10^(-2.00)=0.01M](https://img.qammunity.org/2021/formulas/chemistry/college/bdwc9fick1bkjf84umywrgrkod1w5ofqse.png)
Which also equals the concentration of
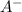 and the in general the ionization extent, therefore, the acid ionization constant, Ka, turns out:
and the in general the ionization extent, therefore, the acid ionization constant, Ka, turns out:
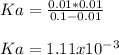
Regards.