Answer:
Sum.
Step-by-step explanation:
Hello,
In this case, the Dalton's law states that the SUM of the partial pressures of each gas in a mixture must equal the total pressure of the entire gas mixture, considering its mathematical definition for a i-containing gas mixture:
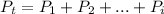
Regards.