Answer:
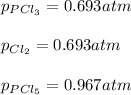
Step-by-step explanation:
Hello,
In this case, for the given reaction, the equilibrium expression is:
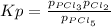
But in terms of the reaction extent
 can also be written as:
can also be written as:
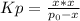
Whereas the initial pressure is 1.66 atm. Thus, we write:
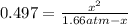
And solving for
 we obtain:
we obtain:
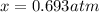
Therefore, the pressure of each species at equilibrium is:
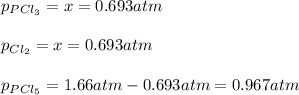
Best regards.