Answer:
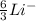
Step-by-step explanation:
The number of protons and neutrons together create total atomic mass.
The number of protons determine what element it is on the Periodic Table.
The number of electrons v protons determine the charge of the atom.
Protons are positive, electrons are negative, neutrons have no charge.
Since there is 1 more electron than protons, the overall charge is going to be -1.
We are given that there are 3 protons, so that gives us the Lithium element with a -1 charge.
Add up the 3 protons with the 3 neutrons and we get 6 as the total atomic mass in this atom.