Answer:
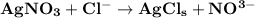
Step-by-step explanation:
The addition of AgNO3 to the system Cl⁻ react with result into the formation a precipitate of AgNO3, as such the reactant concentration decreases.
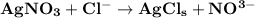
Suppose the concentration of a given substance is present in an equilibrium system is changed without a change in any of the other conditions, then, by le chatelier's principle, the position of equilibrium will move to decrease the concentration of the added substances. In order to relieve this constraint, the equilibrium will shift to the right favouring the forward reaction.