Answer:
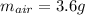
Step-by-step explanation:
Hello,
In this case, given the law of conservation of mass which states that mass cannot be created nor destroyed, since the produced mass of the compound is 15.9 g and the mass of the initial substance is 12.3 g we obtain the mass of air by applying the following equation:
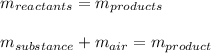
Thus, solving for the mass of air we obtain:
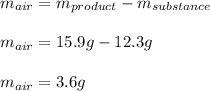
Regards.