Given :
Initial mass , a = 150 gm .
Half time ,
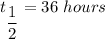 .
.
Final mass , x = 18.75 gm .
To Find :
The time taken to decay 18.75 gm .
Solution :
We know , time taken is given by :
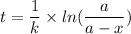
Here , k is a constant given by :
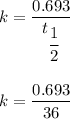
Putting all given value in above equation :
We get :
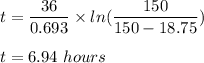
Therefore , time taken is 6.94 hours .
Hence , this is the required solution .