Answer:
Maximum pressure P = 4.9 × 10⁻⁵ Pa
Step-by-step explanation:
From the information given, the mean free path can be expressed with the formula:
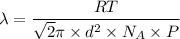
Making Pressure P the subject of the formula because we intend to find the maximum pressure, we have:
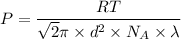
At standard conditions
R = gas constant = 8.314 J/mol.K
T = temperature at 25°C = (273 + 25) = 298 K
π = pi = 3.14
d = (364× 10⁻¹²m)²
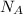 = avogadro's number = 6.023 × 10²³
= avogadro's number = 6.023 × 10²³
λ = mean free path = 1.0 m
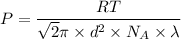
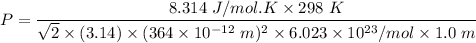
P = 0.007 kg/m.s²
P = 0.007 Pa
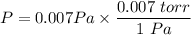
P = 4.9 × 10⁻⁵ Pa