Answer:
Step-by-step explanation:
Relation between ΔG₀ and K ( equilibrium constant ) is as follows .
lnK = - ΔG₀ / RT
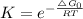
The value of R and T are same for all reactions .
So higher the value of negative ΔG₀ , higher will be the value of K .
Mg(s) + N₂0(g) → MgO(s) + N₂(g)
has the ΔG₀ value of -673 kJ which is highest negative value . So this reaction will have highest value of equilibrium constant K .