Answer:
a. 8.31 x 10-5 s-1
Step-by-step explanation:
The general first-order reaction is:
ln[A] = ln[A]₀ -kt
Where [A] is acutal concentration of reactant, initial concentration is [A]₀, k is rate constant and t is time pass
And the equation of the half-life, t 1/2, is:
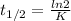
Has half-life is 139min:
139min * (60s / 1min) = 8340s
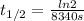
Half-life is 8.31x10⁻⁵ s⁻¹
a. 8.31 x 10-5 s-1