Answer:
0.4 ml will be affect the density.
Step-by-step explanation:
Given that,
A student filled the graduated cylinder up to the 9.6 mL mark.
Recorded volume = 10.0 mL
We know that,
Density :
Density is equal to the mass divided by volume.
In mathematically,
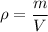
Where, m = mass
V = volume
Affect the density :
Affect the density is equal to the change in volume.
We need to calculate the affect the density
Using formula of affect the density
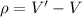
Put the value into the formula
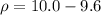
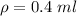
Hence, 0.4 ml will be affect the density.