Answer:
-
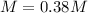
-
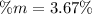
Step-by-step explanation:
Hello,
In this case, since the molar mass of potassium nitrate is 101.1 g/mol, we can compute the molarity as follows:
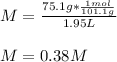
Moreover, as the mass percent is computed as:
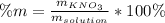
Thus, by using the given density of the solution, we obtain:
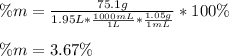
Regards.