Answer : The volume of liquid is 420 mL.
Explanation :
Density : The mass per unit volume of a substance is known as density.
Formula used:
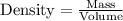
As we are given:
Density of mercury = 13.5 g/mL
Mass = 12.5 pounds
First we have to convert mass of sample from pound to gram.
Conversion used:
As, 1 pound = 453.6 g
So, 12.5 pounds = 453.6 × 12.5 g = 5670 g
Now we have to calculate the volume of liquid.
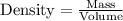
Now putting all the given values in this formula, we get:
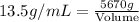
Volume = 420 mL
Therefore, the volume of liquid is 420 mL.