Answer:
a. the minimum amplitude of vibration for the NO molecule A
 4.9378 pm
4.9378 pm
b. the minimum amplitude of vibration for the HCl molecule A
 10.9336 pm
10.9336 pm
Step-by-step explanation:
Given that:
The effective spring constant describing the potential energy of the HBr molecule is 410 N/m
The effective spring constant describing the potential energy of the NO molecule is 1530 N/m
To calculate the minimum amplitude of vibration for the NO molecule, we use the formula:
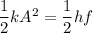
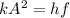
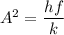
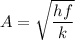
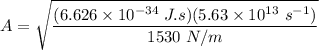
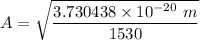
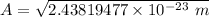
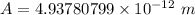
A
 4.9378 pm
4.9378 pm
The effective spring constant describing the potential energy of the HCl molecule is 480 N/m
To calculate the minimum amplitude using the same formula above, we have:
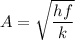
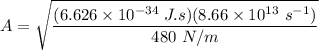
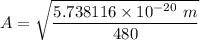
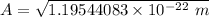
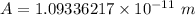
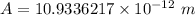
A
 10.9336 pm
10.9336 pm