Answer:
There are
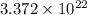 molecules in a cubic centimeter of water at ordinary pressure and temperature.
molecules in a cubic centimeter of water at ordinary pressure and temperature.
Step-by-step explanation:
Let suppose that liquid is water at a pressure of a atmosphere and a temperature of 25 ºC. Due to incompresibility of liquids, water density does not have any change of importance due to changes in pressure and temperature.
Density and molar mass of water are 1 gram per cubic centimeter and 18.015 grams per mole. The mass of water in a cubic centimeter (
 ), measured in grams, is:
), measured in grams, is:
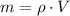
Where:
 - Density, measured in grams per cubic meter.
- Density, measured in grams per cubic meter.
 - Volume of the sample, measured in cubic meters.
- Volume of the sample, measured in cubic meters.
Given that
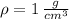 and
and
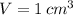 , the mass of water is:
, the mass of water is:
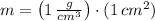
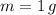
The amount of moles (
 ) inside the sample is:
) inside the sample is:
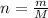
Where:
 - Mass of the sample, measured in grams.
- Mass of the sample, measured in grams.
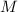 - Molar mass, measured in grams per mole.
- Molar mass, measured in grams per mole.
If
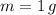 and
and
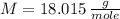 , then:
, then:
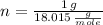
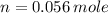
According to the Avogadro's Principle, there are
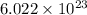 molecules per mole. Hence, the number of molecules in a cubic centimeter of water at ordinary pressure and temperature is determined by simple rule of three:
molecules per mole. Hence, the number of molecules in a cubic centimeter of water at ordinary pressure and temperature is determined by simple rule of three:
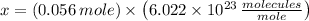
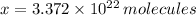
There are
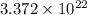 molecules in a cubic centimeter of water at ordinary pressure and temperature.
molecules in a cubic centimeter of water at ordinary pressure and temperature.