Answer:
Second order
Step-by-step explanation:
Given that:
When the reaction A → B + C is studied, a plot 1/[A]t vs. time gives a straight line with a positive slope.
From the integration method for the second order of reaction.
Suppose that:
rate = k₂[A]²
∴
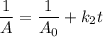
Therefore, a plot of the linear function
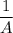 versus t will be linear with a positive slope k₂ and the intercept on the concentration axis will be
versus t will be linear with a positive slope k₂ and the intercept on the concentration axis will be
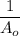
The linear plot for a second order reaction can be seen in theimage attached below.