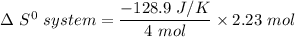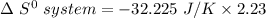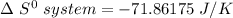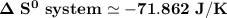Answer:
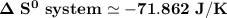
Step-by-step explanation:
The chemical equation for the reaction is given as:
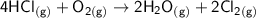
the entropy change in the system can be calculated as follows:
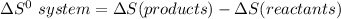
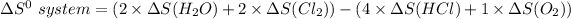
From the tables; the entropy values where obtained.
∴
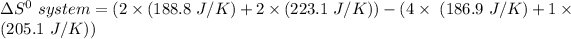
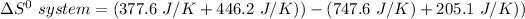
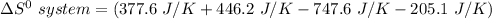
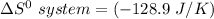
i.e the entropy change in the system when 4 moles of HCl is used = -128.9 J/K
∴
when 2.23 moles of HCl is used, Then,