Answer:
The concentration of
 is 1.48 ×
is 1.48 ×
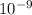 M
M
The absolute uncertainty of
![[{H^(+)]](https://img.qammunity.org/2021/formulas/chemistry/college/4yu59u08dk9ef5g0ktwzyqn2extw55xuqi.png) is ±0.12 ×
is ±0.12 ×
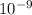 M
M
The concentration of
 is written as 1.48(±0.12) ×
is written as 1.48(±0.12) ×
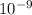 M
M
Step-by-step explanation:
The pH of a solution is given by the formula below
pH =
![-log_(10)[{H^(+)]](https://img.qammunity.org/2021/formulas/chemistry/college/t878r988njaaqkfkwz1x1of7u3xze1ac55.png)
∴
![[H^(+)] = 10^(-pH)](https://img.qammunity.org/2021/formulas/chemistry/college/mu7wp3idlhdertovt8dzmf0scmdyfo090w.png)
where
![[{H^(+)]](https://img.qammunity.org/2021/formulas/chemistry/college/4yu59u08dk9ef5g0ktwzyqn2extw55xuqi.png) is the
is the
 concentration
concentration
From the question,
pH = 8.83±0.04
That is,
pH =8.83 and the uncertainty is ±0.04
First, we will determine
![[{H^(+)]](https://img.qammunity.org/2021/formulas/chemistry/college/4yu59u08dk9ef5g0ktwzyqn2extw55xuqi.png) from
from
![[H^(+)] = 10^(-pH)](https://img.qammunity.org/2021/formulas/chemistry/college/mu7wp3idlhdertovt8dzmf0scmdyfo090w.png)
![[{H^(+)] = 10^(-8.83)](https://img.qammunity.org/2021/formulas/chemistry/college/ziuyatp8ytq25lsx5z85cfop7ujzhu4rxf.png)
![[{H^(+)] = 1.4791](https://img.qammunity.org/2021/formulas/chemistry/college/5u47fs3kys2qm2a5317nyqa8isuv2m33qb.png) ×
×
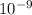 M
M
![[{H^(+)] = 1.48](https://img.qammunity.org/2021/formulas/chemistry/college/typzmfqhphia8tbus4p2u81cccb3rqum6g.png) ×
×
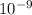 M
M
The concentration of
 is 1.48 ×
is 1.48 ×
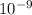 M
M
The uncertainty of
![[{H^(+)]](https://img.qammunity.org/2021/formulas/chemistry/college/4yu59u08dk9ef5g0ktwzyqn2extw55xuqi.png) (
(
![U_([H^(+)] )](https://img.qammunity.org/2021/formulas/chemistry/college/ehkcfkazvqalu3xgqjq1d88boz2uetzjyf.png) ) from the equation
) from the equation
![[H^(+)] = 10^(-pH)](https://img.qammunity.org/2021/formulas/chemistry/college/mu7wp3idlhdertovt8dzmf0scmdyfo090w.png) is
is
![U_([H^(+)] ) = 2.303 \\](https://img.qammunity.org/2021/formulas/chemistry/college/9bwsi53ng8vdvhb78crzkosfezlw75jg4f.png) ×
×
![{[H^(+)] }](https://img.qammunity.org/2021/formulas/chemistry/college/w9gusm0hb4a1bqcfsx58ld52fkzh7oa9y6.png) ×
×
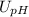
Where
![U_([H^(+)] )](https://img.qammunity.org/2021/formulas/chemistry/college/ehkcfkazvqalu3xgqjq1d88boz2uetzjyf.png) is the uncertainty of
is the uncertainty of
![[{H^(+)]](https://img.qammunity.org/2021/formulas/chemistry/college/4yu59u08dk9ef5g0ktwzyqn2extw55xuqi.png)
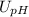 is the uncertainty of the pH
is the uncertainty of the pH
Hence,
![U_([H^(+)] )](https://img.qammunity.org/2021/formulas/chemistry/college/ehkcfkazvqalu3xgqjq1d88boz2uetzjyf.png) = 2.303 × 1.4791 ×
= 2.303 × 1.4791 ×
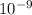 × 0.04
× 0.04
![U_([H^(+)] )](https://img.qammunity.org/2021/formulas/chemistry/college/ehkcfkazvqalu3xgqjq1d88boz2uetzjyf.png) = 1.36 ×
= 1.36 ×
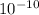 M
M
![U_([H^(+)] )](https://img.qammunity.org/2021/formulas/chemistry/college/ehkcfkazvqalu3xgqjq1d88boz2uetzjyf.png) = 0.12 ×
= 0.12 ×
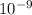 M
M
Hence, the absolute uncertainty of
![[{H^(+)]](https://img.qammunity.org/2021/formulas/chemistry/college/4yu59u08dk9ef5g0ktwzyqn2extw55xuqi.png) is ±0.12 ×
is ±0.12 ×
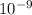 M
M