Answer:
The daughter nuclides of these two decay processes are
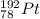 and
and
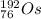 .
.
Step-by-step explanation:
The beta emission is represented by:
A = (Z + 1) + (n - 1) = is invariant
n: neutron
p: proton
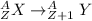
Hence, the daughter nuclide of the beta emission of Ir-192 is:
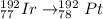
Now, electron capture is represented by:
A = (Z - 1) + (n + 1) = is invariant
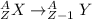
Then, the daughter nuclide of the electron capture of Ir-192 is:
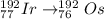
Therefore, the daughter nuclides of these two decay processes are
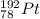 and
and
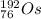 .
.
I hope it helps you!