Answer:
The correct answer is : 1.07 lbs
Step-by-step explanation:
solution:
molar mass glucose (C6H12O6) = 180 g/mol
molar mass of oxygen molecule (O2) = 32 g/mol (as we know molar mass of O = 16 g/mol)
the balanced reaction of conversion of water and oxygen to glucose is:
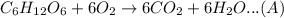
1 mol of C6H12O6 = 6 mol of O2 (from reaction A)
so, 180 g C6H12O6 = 192 g O2
that is, 0.396 lb of C6H12O6 = 0.423 lb of O2 ( 1 g = 0.00220462 lb )
so,
1 lb C6H12O6 =
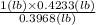 = 1.07 lb O2
= 1.07 lb O2
therefore, the correct answer is : 1.07 lbs