Answer:
Density = 1.59 g/mL
Step-by-step explanation:
The density of carbon tetrachloride can be found by using the formula
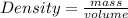
From the question
Volume = 225 mL
To find the mass of carbon tetrachloride in the flask subtract the mass of the flask from the total mass of the flask and carbon tetrachloride
That's
mass of carbon tetrachloride =
703.55 - 345.8
= 357.75 g
Substitute the values into the above formula and solve for the density
That's
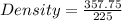
We have the final answer as.
Density = 1.59 g/mL
Hope this helps you