Answer:
47.68 x 10²³ or 4.768 x 10²⁴ copper atoms
Step-by-step explanation:
Given:
Radius of the copper sphere (r) = 0.935 in
First convert the radius from inches to centimeters
1 in = 2.54cm
0.935in = 0.935 x 2.54cm = 2.3749cm
∴ r = 2.3749cm
Calculate the volume of the copper sphere as follows
Volume =
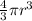 [substitute r = 2.3749cm and π = 22 / 7]
[substitute r = 2.3749cm and π = 22 / 7]
Volume =
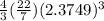
Volume = 56.108cm³
From the volume and given density, calculate the mass of the copper sphere
mass = density x volume [density = 8.96g/cm³]
mass = 8.96 x 56.108 = 502.73g
From known facts
1 mole of copper = 63.5g of copper = 6.022 x 10²³ copper atoms.
Then,
502.73 g of copper =
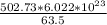 = 47.68 x 10²³ copper atoms
= 47.68 x 10²³ copper atoms
Therefore, the sphere contains 47.68 x 10²³ copper atoms