Answer:
The chemical reaction releases 23400 kilojoules due to 1.5 kg-reactant.
Step-by-step explanation:
Given the amount of energy released due to reactant consumption, total energy (
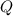 ), measured in kilojoules, is determined by the following expression:
), measured in kilojoules, is determined by the following expression:
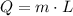
Where:
 - Mass of reactant, measured in kilograms.
- Mass of reactant, measured in kilograms.
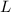 - Released heat ratio, measured in kilojoules per gram.
- Released heat ratio, measured in kilojoules per gram.
Given that
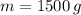 and
and
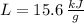 , the total energy released by chemical reaction is:
, the total energy released by chemical reaction is:
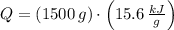
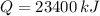
The chemical reaction releases 23400 kilojoules due to 1.5 kg-reactant.