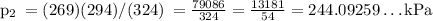Answer:
• 1.62432 moles of nitrogen
• Tire Pressure: 2.74 * 10⁵ Pa
• The tires will burst
• Pressure: 244 kPa
Step-by-step explanation:
• We can determine the number of moles of nitrogen using the formula pV = nRT, where p = pressure, V = volume, n = number of moles, R = gas constant, and T = absolute temperature.
Now remember we have our initial pressure in kilopascals so let's convert to pascals (249 pascals). The volume is given in liters, so let's convert into m². And the initial temperature is given in Celsius ⇒ our absolute temperature in Kelvins.
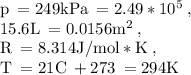
Respectively the moles of nitrogen in each tire should be:
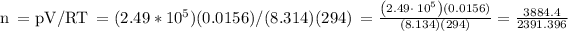
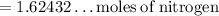
• We can solve this part similarly. All our values will be the same, besides the temperature, as we have to consider both the initial and final temperature here.
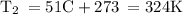 -
-
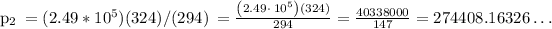
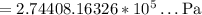
• The text mentions that the tires will burst when the internal pressure reaches 269kP. From part #2 we know that the final pressure will be, in kilopascals, 274kP. As 274 > 269, the tires will burst in Death Valley.
• We would want the final temperature = breaking pressure. Therefore,