This is an exercise of Equation of state of an ideal gas.
To start solving this exercise, we obtain the following data:
DATA:
- V₁ = 2.0 l
- P₁ = 1.0 atm
- V₂ = 0.25 l
- P₂ = ¿?
The gas law is derived from the gas law of Boyle, Charles, Gay Lussac and General.
Boyle's law says:
- "At constant temperature, the volume of a fixed mass of gas is inversely proportional to the pressure it exerts."
This means that if the volume is doubled the pressure is reduced by half, and if the pressure is made three times greater, the volume will be one third of what it was originally which can be summarized in the equation.
P₁V₁=P₂V₂ ⇒ General Formula
Where:
- P₁: Initial pressure
- V₂: Initial volume
- P₂: Final pressure
- V₂: Final Volume
The word inversely proportional means: "That if the pressure increases the volume decreases but proportionally".
We clear formula for final pressure:
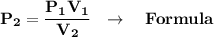
We clear our data in the formula:
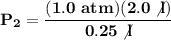
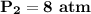
If the volume is reduced to 0.25 liters, the new pressure of the gas will be 8 atm.
{ Pisces04 }