Answer:
The equivalent weight of M is approximately 31.8 g
The equivalent weight of N is approximately 27.98 g
Step-by-step explanation:
The given parameters are;
The percentage of the the metal M in in the chloride = 47.25%
Where by the chemical formula for the metal chloride is MClₓ, we have;
47.25% of the mass of MClₓ = Mass of M = W
Therefore, we have;
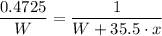
0.4725 × (W + 35.5·x) = W
0.4725·W + 0.4725×35.5×x = W
W - 0.4725·W = 16.77·x
0.5275·W = 16.77·x
W/x = 16.77/0.5275 = 31.799 = The equivalent weight of M
The equivalent weight of M = 31.799 ≈ 31.8 g
Given that 1 gram of M is displaced by 0.88 gram of N, then the equivalent weight of N that will displace 31.799 = 0.88 × 31.799 ≈ 27.98 g
The equivalent weight of N = 27.98 g.