Answer:
Molarity = 7 mol / L
Step-by-step explanation:
Since the mass of NaCl and it's volume has been given we can find the molarity by using the formula
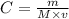
where
C is the molarity
m is the mass
M is the molar mass
v is the volume
From the question
v = 0.5 L
m = 205 g
We must first find the molar mass and then substitute the values into the above formula
M( Na) = 23 , M( Cl) = 35.5
Molar mass of NaCl = 23 + 35.5 =
58.5 g/mol
So the molarity of NaCl is
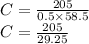
C = 7.00854
We have the final answer as
Molarity = 7 mol / L
Hope this helps you