Answer:
The answer is
1.20 L
Step-by-step explanation:
Since the concentration , mass and molar mass of NaOH has been given we use the formula
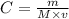
To find the volume
where
C is the concentration
m is the mass
M is the molar mass
v is the volume
Making volume the subject we have
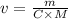
From the question
C = 1.25 M
m = 60 g
M = 40.0 g/mol
Substitute the values into the above formula and solve for the volume
We have
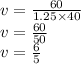
We have the final answer as
Volume = 1.20 L
Hope this helps you